Natural zeolites and synthetic A and X-type zeolite molecular sieves are mainly used in industry for adsorption separation and ion exchange.
Among them, NaA or NaX zeolites can be modified by ion exchange with metal ions such as alkali metals or alkaline earth metals to improve their adsorption and separation performance on gas mixtures. Y-type zeolites and heteroatomic molecular sieves formed by replacing silicon or aluminum on the zeolite framework with other atoms (such as P, B, Sn, Ti, Ga, etc.) are mainly used as catalysts.
The types of zeolite molecular sieves commonly used as adsorbents include A-type and X-type molecular sieves.
Now let’s talk about A type molecular sieve:
1, what is A type molecular sieve?
A-type (LTA) molecular sieves (3A, 4A, 5A)
The topology of type A zeolite is LTA type, and its silica-alumina ratio is 1.0. The β-cage (or calcite cage) in the cell structure is in octahedral coordination, and the α-cage is formed by interlinking four oxygen atoms between the β-cages. The free diameter of the cell (α-cage) is about 1.14 nm, and the effective volume is 0.76 nm3, and there are six main crystal pores of the octahedral ring, and its free diameter is 0.44 nm. In each type A zeolite cell contains 12 cations with balanced negative charge, and the effective pore size of the main crystalline pore in the zeolite framework is affected by the cations in the crystal lattice.
- 4A(NaA) molecular sieve is a synthetic A-type zeolite with Na+ cations, and the effective pore size of the main pore is about 0.38nm.The typical cell composition is Na12 [(AlO2)12 (SiO2)12]-27H2O;
- 3A (KA) molecular sieve is a 4A zeolite in which the Na+ ion is replaced by K+ with a larger ionic radius, and the effective pore size of the main pore will be reduced to 0.30 nm, and the typical cell composition is K12 [(AlO2)12 (SiO2)12]-27H2O;
- 5A (CaA) molecular sieve is obtained by exchanging 4A zeolite with Ca2+, where 2 Na+ are replaced by 1 Ca2+ and the effective pore size increases to 0.43 nm. and the typical cell composition is Ca5Na2[(AlO2)12 (SiO2)12]·27H2O.
In this way, the prepared 3A, 4A and 5A are molecular sieves with different effective pore sizes, which molecules of different sizes can be sieved, i.e., molecules smaller than the effective pore size can enter the active surface of the cell and be separated by adsorption, while molecules larger than the pore size are isolated outside the micropores.
2, 4A molecular sieve
The unique adsorption properties of 4A zeolite are easily highlighted by the exchange between cations, as shown in Figure 1.
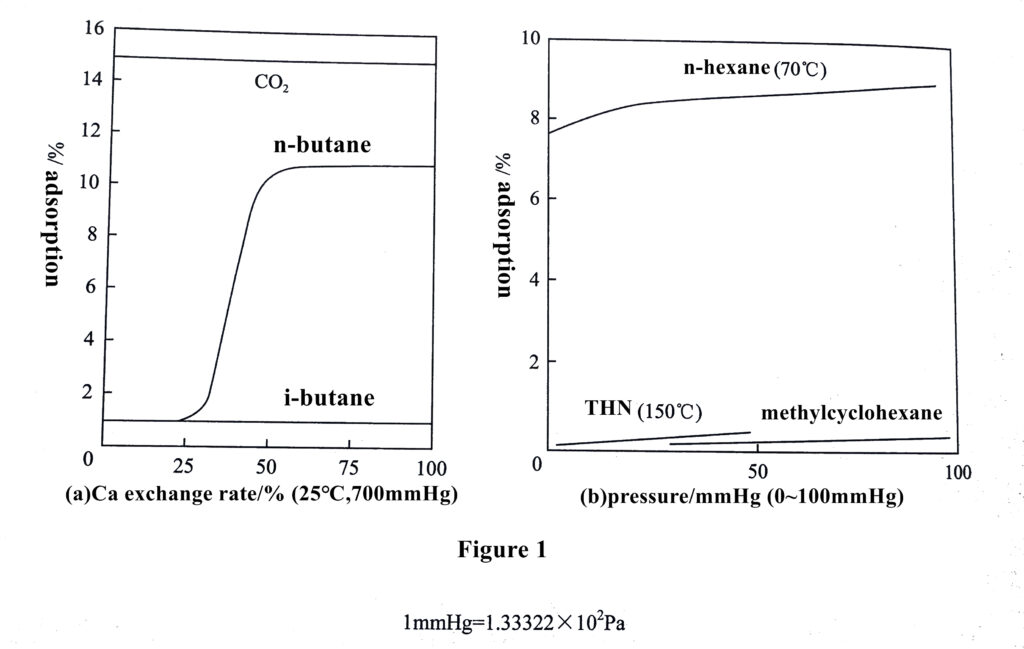
Figure 1-11 (a) shows the effect of the change in pore size and adsorption force on the adsorption of different molecules (CO2, n-butane, iso-butane) with increasing Ca2+ exchange degree.
Among them, the pore size of 4A zeolite is 0.38 nm, and carbon dioxide molecules (molecular kinetic diameter of 0.33 nm) can enter, while n-butane with a larger diameter of 0.49 nm and iso-butane with 0.56 nm cannot enter the adsorbent, so no adsorption is shown when the Ca2+ exchange degree is low.
3, 5A molecular sieve
when Na+ was exchanged by Ca2+ in 1/3 amount, the adsorption amount of n-butane increased sharply, which was due to the reduction of the number of cations in the zeolite after Ca2+ exchange, and the vacancy position was vacated, so that the zeolite pore size could become larger to 0.50 nm; meanwhile, the adsorption data showed that the larger size of iso-butane molecules still could not be adsorbed, so the zeolite molecular sieve with this size pore size could separate n-butane and iso-butane mixture very well.
In addition, Figure 1(b) shows the adsorption results of n-hexane (0.49 nm in diameter) and a mixture of benzene, tetrahydronaphthalene(THN), and methylcyclohexane with molecular diameters larger than 0.5 nm on the 5A molecular sieve. As shown in the figure, the adsorption of n-hexane by the 5A molecular sieve was significantly higher than the other two molecules, indicating that this zeolite can selectively adsorb n-hexane and exclude adsorbent masses with larger molecular sizes from the adsorbent pore channel. It was also found that the Ca2+exchanged 5A zeolite selectively adsorbed n-butanol and higher n-alcohols, n-butene and higher n-olefins, propane and C4-C14 n-alkanes and cyclopropane. These studies have shown that efficient separation of target mixtures can be achieved by rational adjustment of zeolite pore size through ion exchange of zeolites.
In addition, researchers found that the presence of Ca2+ in the framework of 5A zeolite can promote its high adsorption selectivity for polar molecules and unsaturated compounds. 5A molecular sieve has a strong affinity for water and shows great advantages over other desiccants, even under a low water pressure environment (e.g., water pressure of 2X10-4mmHg), the water absorption of 5A molecular sieve as a desiccant is as high as 14.0%, which is significantly better than other desiccants. 14.0%, which is obviously better than other desiccants such as 4A (10.3%), 13X (11.7%) and Al2O3 (2.0%)].
Moreover, under the higher temperature, the water adsorption of 5A molecular sieve can still reach 13% under the condition of 100°C, and even under the condition of 200°C, it can still retain 4% of the water adsorption, while the water adsorption of silica gel, Al2O3, and other important desiccants under the same high temperature condition of 100°C is close to zero.
In addition, in the high-speed gasflow environment, 5A molecular sieve still maintains a fairly high water absorption efficiency. Based on the above characteristics, 5A molecular sieve has been used as an important desiccant for rare gases and permanent gases in industrial raw materials, and as a dehydrating desiccant for medium and high pressure air.
Meanwhile, 5A molecular sieve can quickly achieve adsorption equilibrium for gas adsorption, and the adsorption volume is large and the separation effect is remarkable, so 5A is suitable for both pressure swing adsorption separation, such as for N2-H2 separation, N2-He separation, hydrogen purification, air separation and oxygen production, etc.; it can also be used for temperature swing adsorption separation, such as extracting helium from natural gas by deep cooling method, in which 5A molecular sieve is used for adsorption and removal of carbon dioxide, etc. . In view of the excellent performance in the above studies, 5A molecular sieve has been used in large quantities as an important adsorption material in industry, and is widely used in the industrial adsorption and separation territory for the separation or purification of liquids and gases.

4, 3A molecular sieve
3A molecular sieves obtained by ion exchange with K+, which has a larger ionic radius, have smaller pore size and are currently mainly used in the drying of petroleum cracking gas and natural gas.
LPG contains a large number of hydrocarbons, especially olefins, which are adsorbed in the micro-pores of general adsorbents during the drying process, polymerizing and cracking into coke, blocking the pore channels, thus reducing the adsorption performance and shortening the service life. 3A molecular sieve for drying LPG does not have these disadvantages.
Because of its small and uniform pore size, it can adsorb only water, but not hydrocarbons with larger molecules. In the drying process of petroleum cracking gas, compared with other desiccants (such as silica gel, low density and high density alumina and other adsorbent materials), 3A molecular sieve can fully demonstrate its superiority.
For example, in drying petroleum gas and olefin deep cooling separation raw gas, 3A molecular sieve fully demonstrates its superiority of selective adsorption of water but not olefin, and is a widely used excellent desiccant
