What is Adsorption equilibrium
There is a dynamic equilibrium between adsorption and desorption of adsorbate molecules on the adsorbent surface (or pore channel). The adsorption equilibrium is the dynamic equilibrium state formed by the equal amount of adsorbed and desorbed adsorbate molecules under certain conditions.
The adsorption equilibrium is relative and conditional, formed under certain temperature and pressure conditions, and once the conditions change, the original equilibrium will be broken and a new equilibrium relationship will be established. The adsorption amount of a substance is the adsorption amount under the adsorption equilibrium condition, which is related to the adsorbent, adsorbate molecules and adsorption conditions, the conditions are certain, the adsorption amount is certain;
The adsorption amount is an important index to evaluate and measure the adsorption performance of the adsorbent.

The adsorption amount of adsorbate gas on the adsorbent is a function of temperature, pressure and affinity or action energy. For the adsorption system, the phase law can be used to calculate the degree of freedom of the adsorption equilibrium system.
Since there is an independent adsorption phase between the adsorbate and the adsorbent, the degrees of freedom of the adsorption equilibrium system = number of independent groups + number of phases -2. For the adsorption equilibrium of a single component, the number of independent groups is 2 (adsorbate and adsorbent) and the number of phases is 3 (fluid phase, solid phase and adsorption phase), then the number of degrees of freedom of the single component adsorption equilibrium is 3 (adsorbent, temperature and pressure); therefore, the equilibrium adsorption amount will vary with temperature, pressure and adsorbent, i.e., the equilibrium adsorption amount is related to the temperature of the system, the pressure of the gas and the characteristics of the interaction between the gas and the solid.
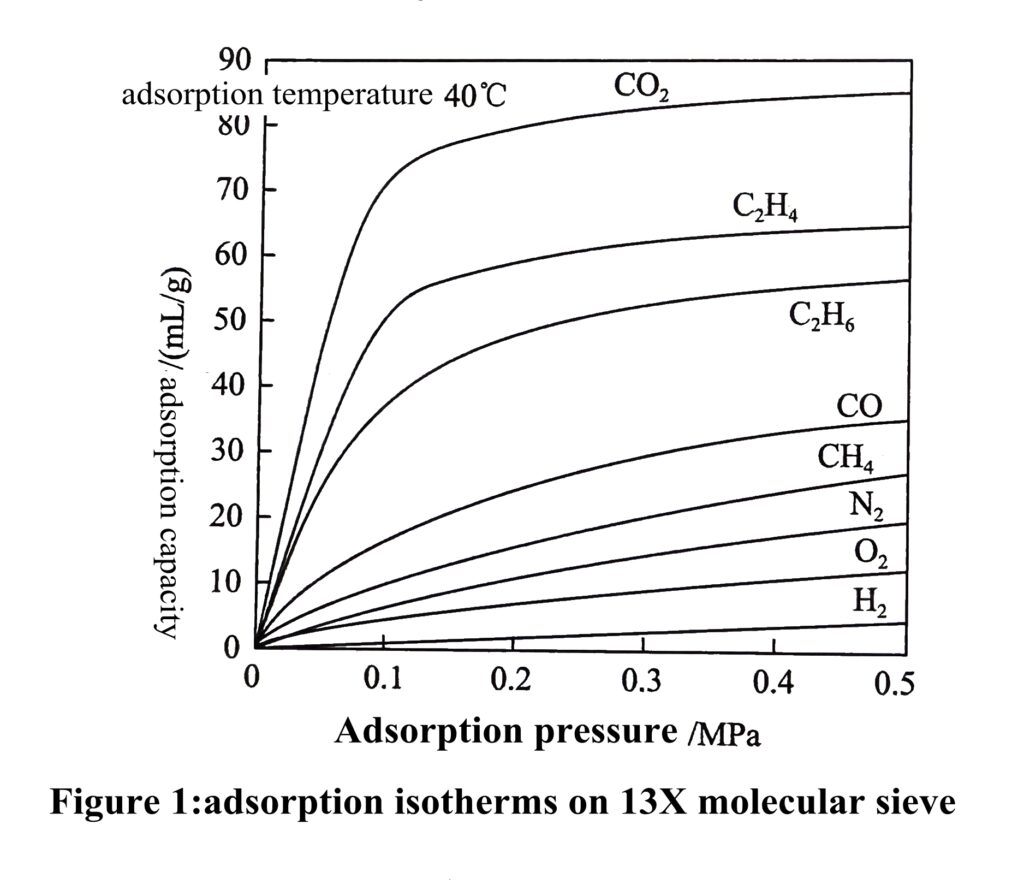
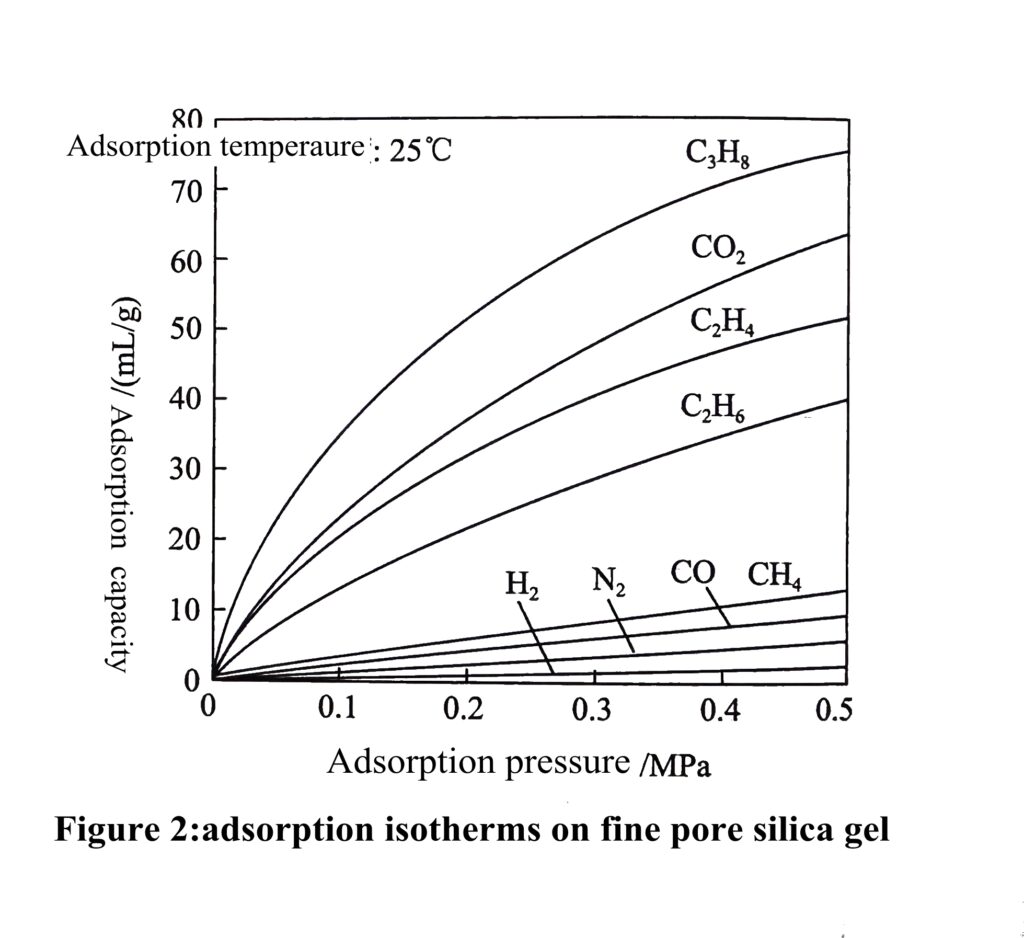
For a given gas-solid system, the equilibrium adsorption amount of the gas is only related to the temperature and pressure, and the adsorption amount at equilibrium can be expressed intuitively as q=f(p,T), where q is the equilibrium adsorption amount: at a certain temperature T, q is only a function of the pressure p. At this point, the curve expressing the correspondence between equilibrium adsorption amount and pressure is called adsorption isotherm, while the curve expressing the equilibrium adsorption amount at a certain pressure versus temperature is called adsorption isobaric; where adsorption isotherm is more commonly used, the adsorption isotherms of some conventional gases on 13X molecular sieve and fine pore silica gel are shown in Figure 1 and 2, respectively
Contact Libra for more support

