We have talked about A type molecular sieve, now we will move on to X type molecular sieve:
1, what is X type molecular sieve
X-type (FAU) molecular sieves (13X, 10X, NaLSX, LiLSX, CaLSX)
X-type zeolite molecular sieves have FAU crystal skeleton structure, the total number of silicon and aluminum atoms in each cell is 192, that is, 192 silicon aluminum oxygen tetrahedra, only the silicon to aluminum atom ratio is different.
X-type zeolite cell structure silicon aluminum ratio is 1.0~1.25, in which each cell contains 96~77 aluminum ions. The β-cages in the structure are linked by six oxygen bridges on the hexagonal column cage in the hexagonal ring to produce a super-cage – octahedral zeolite cage.
The free diameter of the cell (super-cage) is 1.18 nm, and the effective volume is 0.85 nm3. The octahedral zeolite structure has a large central pore volume and about 50% porosity after dehydration; each cell has four main crystalline pores in the dodecahedral ring, and the free diameter of the main crystal pore is 0.74 nm.
2, different types of X molecular sieve
There are five main types of commercial X-type zeolites, namely
- 13X (NaX),
- 10x (CaX),
- NaLSx,
- LiLSX
- CaLSX,
which involve three main cations, Na+, Li+ and Ca2+, depending on the silicon-alumina ratio and the type of cations contained in the zeolite.
- the silicon-aluminum ratio of 13X zeolite molecular sieve is about 1.23, and the typical cell composition is Na86 [(AlO2) 86 (SiO2)106]-264H2O;
- the silica-alumina ratio of NaLSX zeolite is about 1.0, and its synthesis process conditions are different from those of 13X molecular sieve, and the silicon-aluminum ratio is more strictly controlled and the preparation time is longer,
- there are two types of X-type zeolites generated by Ca2+ exchange,
- 10X molecular sieve is made by replacing Na+ in 13X by Ca2+ exchange, with the same silicon-aluminum ratio as 13X, and the cell composition can be Ca34Na18 [(AlO2) 86 (SiO2) 86 (SiO2)] -264H2O. AlO2) 86 (SiO2)106]-264H2O (Ca2+ exchange degree 79%).
- CaLSX zeolite (low-Si 10X) is substituted for Na+ in NaLSX by Ca2+ exchange, and the composition in the cell can be Ca48-xNa2x [(AlO2) 96 (SiO2)96]-264H2O, in which Ca2+ exchange can reach more than 96%, and the adsorption separation performance is more outstanding.
- Li+ is exchanged with Na+ (with a small amount of K+) in NaLSX zeolite to prepare LiLSX molecular sieve, i.e. LiX molecular sieve.
The NaLSX, LiLSX and CaLSX zeolites introduced above are all low-silica zeolite molecular sieves, which have higher adsorption and separation performance than the conventional X-type zeolite molecular sieves.
Since the 1970s, 13X has been used commercially in air separation, and NaLSX was developed in the early 1980s, followed by commercial LiLSX molecular sieves prepared by Li+ exchange; so far, LiLSX molecular sieves are the best commercial adsorbents for PSA air separation.

3, why LiX molecular sieve is widely used?
In the 1980s, studies on the preparation of LiX (silicon-to-aluminum ratio 1.0) molecular sieves by Li+ exchange found that:
(i) the nitrogen adsorption capacity shows a significant increase only when the Li+ exchange degree reaches 70% in LSX (silicon-to-aluminum ratio 1.0) zeolite or 80% in 13X zeolite, and it increases linearly with the increase of Li+ exchange degree.
(ii) The nitrogen adsorption increases significantly as the silica-alumina ratio in X zeolite decreases and approaches 1.0. Meanwhile, the study of the relationship between the silicon-to-aluminum ratio and the nitrogen/oxygen separation coefficient α(N2/O2) of lithium molecular sieves at certain adsorption temperatures (250~320 K) and pressures (25~150 kPa) revealed that:
①α(N2/O2) (silicon-to-aluminum ratio=1.0) >α(N2/O2) (silicon-to-aluminum ratio=1.15) >α(N2/O2) (silicon-to-aluminum ratio=1.25).
And α(N2/O2) (silicon-to-aluminum ratio=1.0) is most prominent at 273K for different pressures, while α(N2/O2) (silicon-to-aluminum ratio=1.15) is most prominent at 300K;
②The effect of temperature on α(N2/O2) is greater than that of pressure, and the best temperature range is from 273 to 300K, and the effect of decreasing temperature is more obvious than that of increasing temperature on the decrease of α(N2/O2).
Like other zeolite molecular sieves, LiLSX molecular sieve has similar adsorption capacity for oxygen and argon, and the adsorption amount increases almost linearly with the increase of pressure, without separation effect; while the adsorption capacity for nitrogen is particularly outstanding, and the effective separation of nitrogen and oxygen (with argon) can be achieved. This is because the smaller the silicon-to-aluminum ratio, the higher the aluminum content in the zeolite skeleton, the more negative charges, the more cations required for electroneutrality in the skeleton, so that there will be more cations to exert force on gas molecules; Li+ has a smaller ionic radius and the largest charge density, with a strong quadrupole moment potential, Li+ in lithium molecular sieve has a strong quadrupole moment force with nitrogen, and stronger adsorption.
4, the advantages of LiX molecular sieve
In air separation oxygen production, LiX (silicon-to-aluminum ratio = 1.0, LiLSX) molecular sieve has the following outstanding advantages:
① higher oxygen recovery and oxygen purity;
② less adsorbent usage per unit oxygen yield;
③ significantly lower unit oxygen power consumption, the ratio of high to low pressure for adsorption and desorption is close to 2.0, while the ratio of high to low pressure for 13X generally needs to be greater than 4.0 (Figure 1);
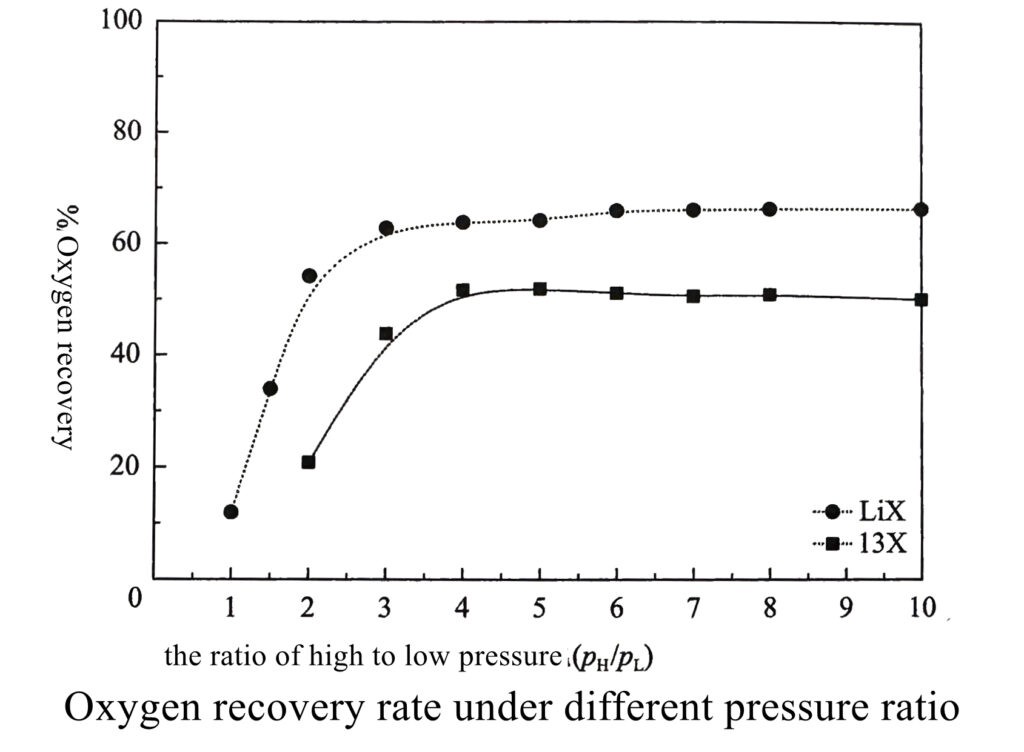
④ suitable for lower adsorption pressure, the requires vacuum desorption.
5, the disadvantages of LiX molecular sieve
However, the outstanding deficiency of LiLSX molecular sieve is the preparation technology requirements and high product prices, the most important aspects of the current production process:
① NaLSX zeolite molecular sieve synthesis, requiring its silicon to aluminum ratio as close as possible to 1.0, and high crystallinity;
② lithium exchange, repeated heating exchange, Li + ion exchange degree is high, generally greater than 96%;
③ using a lower temperature (350 ~ 450 ° C) or high temperature (500~600°C) activation, and after activation and cooling, pure nitrogen is used for packaging and storage.
