For gas purification and separation, Pressure swing adsorption (PSA) and Temperature swing adsorption (TSA) are the main adsorption methods.
Do you know the difference between PSA and TSA? I will show you everything you need to know in this blog:
1, Introduction: Adsorption separation methods
Adsorption and desorption in physical adsorption are characterized by spontaneous, rapid, reversible and dynamic equilibrium.
The equilibrium adsorption capacity of gas in gas-solid system is related to temperature and pressure, and the adsorption isotherm reflects the correspondence between the equilibrium adsorption capacity of gas molecules in the adsorbent and the gas pressure at a certain temperature.
When the adsorption temperature is the same, the adsorption capacity on the adsorbent increases with the rise of adsorbate partial pressure, and the drop of pressure is favorable to adsorbate desorption, i.e. adsorbent regeneration;
in addition, when the adsorbate partial pressure is the same, the adsorption capacity on the adsorbent increases with the fall of adsorption temperature, and the rise of temperature is favorable to adsorbate desorption, i.e. adsorbent regeneration.
To realize the recycling of adsorbent, adsorbent regeneration (i.e. adsorbate desorption) is the key step. According to the desorption mechanism, the adsorbent regeneration method mainly includes four kinds of regeneration:
- pressure reduction,
- heating regeneration,
- flushing regeneration
- and replacement regeneration.
In practical application, pressure reduction and heating are the basis of adsorbent regeneration, while flushing regeneration or replacement regeneration is the supplement and enhancement on this basis, and the whole adsorption separation process is cyclic through adsorbent desorption regeneration.
Gas adsorption and separation can be divided into two categories:
- pressure swing adsorption
- and temperature swing adsorption
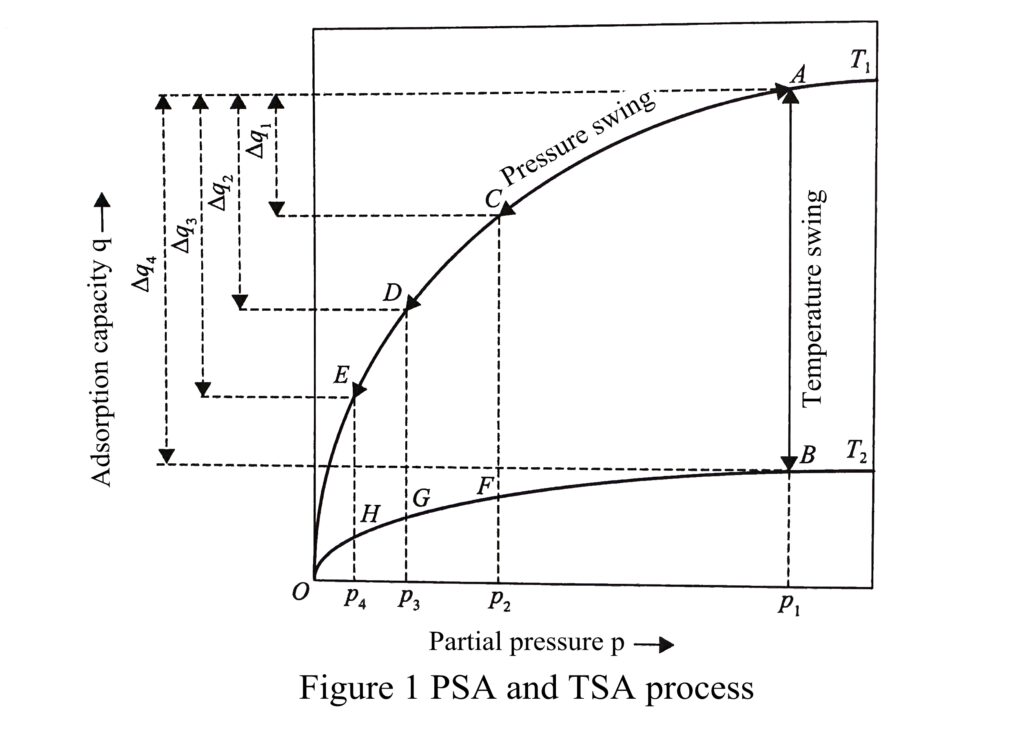
according to the main regeneration methods of the adsorbent, as shown in Figure 1
2, What is PSA?
Pressure swing adsorption (PSA) is a gas adsorption and separation process in which gas components are adsorbed at high pressure and desorbed at low pressure in an adsorption bed.
According to the law that the adsorbent adsorption of adsorbate decreases with the pressure, the adsorption capacity differs significantly at different temperatures and pressures.
The adsorption isotherms of a component on an adsorbent at temperatures T1 and T2 are shown in Figure 1, where T2 > T1. From Figure 1, it can be seen that the adsorption capacity are at points A and C for adsorbent pressures p1 and p2 at temperature T1, respectively.
When the partial pressure of the adsorbate component is lowered from p1 to p2, the desorption capacity is Δq1. If the adsorbate partial pressure is further lowered to p3 and p4 by flushing or vacuum, the corresponding adsorbate adsorption capacity drop to points D and E, respectively. The corresponding adsorbate desorption capacitys are Δq2, Δq3.
The adsorption isotherm reveals the law that the adsorbate adsorption on the adsorbent varies with the rise and fall of pressure. Using this law, the separation of specific components can be accomplished by the pressure swing process of adsorption at elevated pressure and desorption at reduced pressure. After the adsorption process is completed, the partial pressure of adsorbate needs to be reduced as much as possible in order to make adsorbate fully desorbed and the adsorbent completely regenerated.

3, What is TSA?
Temperature swing adsorption (TSA) is a separation process in which gas components are adsorbed at a lower temperature (room temperature or lower) in the adsorption bed and desorbed at a higher temperature.
According to the law that the adsorption of adsorbate decreases with increasing temperature (Figure 1), at a certain pressure, it is known from the adsorption isotherms at different temperatures that by heating the adsorbent from temperature T1 to temperature T2, the capacity of adsorption decreases with increasing temperature, i.e., the capacity of adsorption decreases substantially from point A to point B, and the capacity of desorption is Δq4.
The adsorption isotherms at different temperatures also reveal the law of adsorbate adsorption on the adsorbent with the rise and fall of temperature. Using this law, the separation of specific components can be accomplished by the temperature swing process of low-temperature adsorption and high-temperature desorption.
4, Combination of PSA and TSA
Regeneration of adsorbent is the process of disrupting the existing adsorption equilibrium, restoring its initial adsorption performance and reusing the adsorbent, and the regeneration process requires energy consumption.
If the interaction force between the adsorbent and adsorbate is large, the content of residual adsorbate will still be high after the pressure reduction, which will not meet the adsorption separation process requirements, such as point C in the figure when the desorption pressure is p2;
At this point, the desorption regeneration needs to obtain a high desorption energy to complete.
For the desorption of such components, it is usually necessary to use the heating method to make the residual load reach the point B. Heating regeneration requires energy, but regeneration is more effective.
In some applications, a combination of pressure swing + flushing + temperature swing (e.g., A-C-D-G-B-A in the figure) or pressure swing + vacuum + temperature swing (e.g., A-C-E-H-B-A in the figure) cycle is also used to obtain a very low residual load, which is also a common regeneration process for temperature swing adsorption cycles.
